Company Introduction
Cellid Co.,Ltd. leads research of anti-cancer immunotherapies and Vaccines for preventing infectious diseases
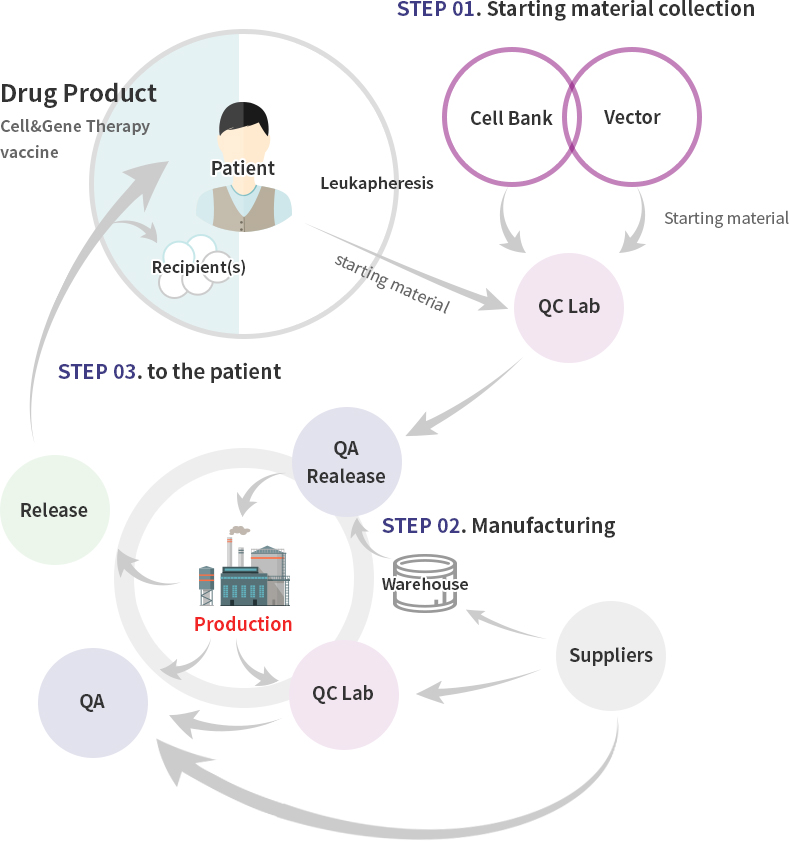
Cellular Gene Medicine GMP Center
CeliVax™ Pipeline is produced under high-level quality system (QMS) suitable for guidelines of Ministry of Food and Drug Safety (MFDS), PIC/S, and Advanced Therapy Medicinal Products (ATMP).
Cellid’s GMP Center is classified into two types.
Cellid’s GMP Center is classified into two types.
- 280 Pyeong (925m2/9963ft2)
- Production facility for complete clinical and commercial medicine
- Faciliy where BSL2 Viral Vector can be used
- Aseptic process room
- Applied with closed system
- Filling and packing
- Storage of complete medicine (LN2 Tank)
- Own QC laboratory
- 187Pyeong(618m2/64454ft2)
- Production facility for complete clinical and commercial medicine
- BSL2 Viral Vector production facility
- Aseptic process room
- Filling and packing
- Cell bank
- Storage of complete medicine
- Own QC laboratory
GMP Manufacturing-infra